Abstract
Introduction. Malignant diseases treated with allogeneic hematopoietic stem cell transplantation (alloHSCT) predominantly occur beyond the 7 th decade of life. Numerical age per se is not regarded an adverse risk factor in alloHSCT. In an aging society, interventions historically deemed high risk are increasingly used in elder patients.
Methods. Epidemiology, outcomes and risk factors of patients aged ≥70 years undergoing alloHSCT in Germany 1999-2019 and registered with the DRST/EBMT database were analyzed retrospectively. Baseline patient, disease, and transplant data were collected from MED-A forms. Centers were contacted to provide additional treatment and follow-up information.
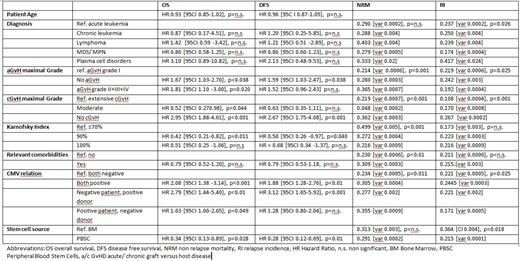
Results. Between 1999 and 2019, 1648 patients aged ≥70 years (median 72, range 70-79.7; 585 female) were transplanted in 50 German centers. More than 90% of all patients were transplanted 2010-2019. Centers transplanted between 2 and 192 patients, with 14 centers contributing <10 and 4 centers contributing >100 patients each. Most patients suffered acute leukemia (1084, 65.8%) or MDS/MPN (410, 24.9%). Karnofsky index before start of conditioning was 100% (n=230, 14%), 90% (n=651, 39.5%), 80% (n=480, 29.1%), 70% (n=94, 5.7%), <70% (n=55, 3.3%). Myeloablative conditioning was chosen in 25.6%. Total body irradiation was used for 305 patients (18.6%). Conditioning contained antithymocyteglobulin in 49.6%. Donors were unrelated for 85.5%. Median donor age was 37 (18-79) years. Patient CMV IgG was positive in 63.1% and the constellation 'negative donor, positive patient' was present in 19.9%. Median overall survival (OS) and disease free survival (DFS) was 408/ 344 days. With a median follow up of 536 days for surviving patients, Kaplan Meier estimates of OS/ DFS were 52.6%/ 48.5% and 40.9%/ 38.6% at 1 and 2 years. In a competing risk analysis, cumulative incidence of non-relapse-mortality (NRM)/ relapse (RI) was 22.2%/ 29.3% at 365 days. Frequency of acute graft versus host disease (GvHD) II-IV was 25.1% and chronic limited/ extended GvHD 11.7%/ 14.8%. Karnofsky performance score, CMV IgG matching, acute and chronic GvHD and stem cell source showed a prognostic impact on OS, DFS, RI and/ or NRM (Table 1). Underlying disease did not impact outcome, neither did age amongst patients at an age of 70-80 years. To compare with outcome in the decade below (60-69 years), an analysis after matching for underlying disease, CMV relation, and Karnofsky index included 2728 patients (each 1364 patients 60-69 and ≥70 years of age). For each year of life, univariate HR for OS and DFS were 1.01 [95%CI 1.001-1.023, p=0.035] and 1.01 [95%CI 0.99-1.02, p=n.s.], respectively, in this matched-pair analysis. The cumulative HR (OS, DFS) for both age groups was 1.16 [95%CI 1.05-1.28, p<0.01] and 1.13 [95%CI 1.02-1.24, p=0.016] for patients ≥70 years.
Conclusion. AlloHSCT is increasingly used to treat elder patients in Germany with a sharp increase during the last decade. Age per se is a modest adverse risk factor for adult patients after alloHSCT with slightly increased mortality in patients 70-80 versus those at 60-69. Further research might concentrate on patient selection and further reduction of procedural toxicity.
Schetelig: Roche: Honoraria, Other: lecture fees; Novartis: Honoraria, Other: lecture fees; BMS: Honoraria, Other: lecture fees; Abbvie: Honoraria, Other: lecture fees; AstraZeneca: Honoraria, Other: lecture fees; Gilead: Honoraria, Other: lecture fees; Janssen: Honoraria, Other: lecture fees . Einsele: Janssen, Celgene/BMS, Amgen, GSK, Sanofi: Consultancy, Honoraria, Research Funding. Stelljes: Pfizer: Consultancy, Research Funding, Speakers Bureau; Medac: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; MSD: Consultancy, Speakers Bureau; Kite/Gilead: Consultancy, Speakers Bureau. Dreger: AbbVie: Consultancy, Speakers Bureau; Bluebird Bio: Consultancy; Novartis: Consultancy, Speakers Bureau; Janssen: Consultancy; AstraZeneca: Consultancy, Speakers Bureau; Gilead Sciences: Consultancy, Speakers Bureau; BMS: Consultancy; Riemser: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Speakers Bureau. Wulf: Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Clinigen: Consultancy, Honoraria. Scheid: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria; Roche: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bethge: Novartis: Consultancy, Honoraria, Speakers Bureau; Kite-Gilead: Consultancy, Honoraria, Speakers Bureau; Miltenyi Biotec: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal